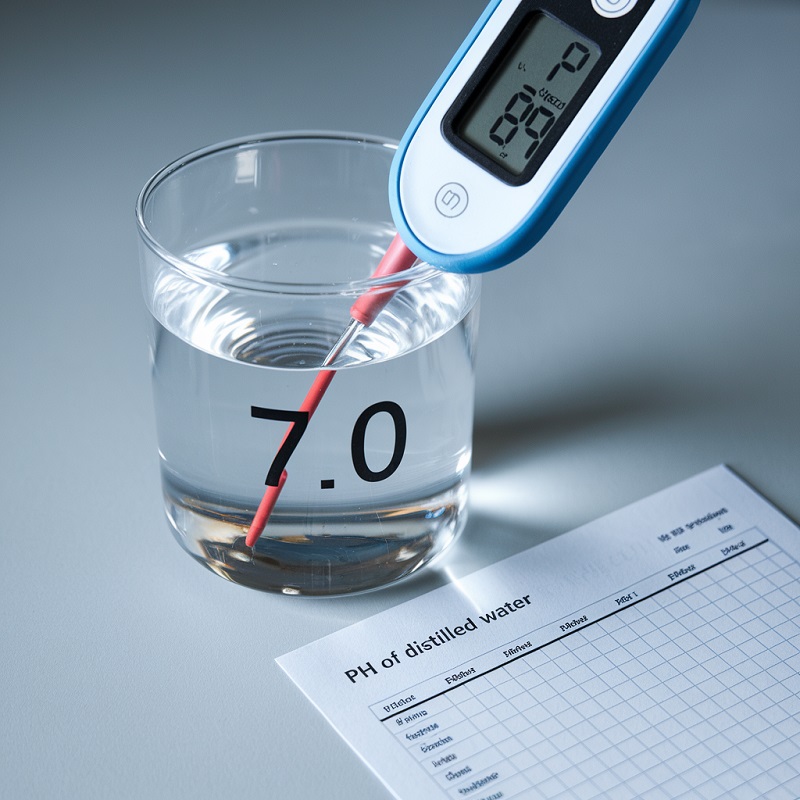
The pH of distilled water refers to its level of acidity or alkalinity on a scale from 0 to 14. Pure distilled water has a pH of 7, which is considered neutral. However, this can change under certain conditions.
What is pH? Why is it an Important Factor for Water and Other Substances?
pH stands for “potential of hydrogen” and measures the concentration of hydrogen ions in a solution. It determines whether a substance is acidic, neutral, or basic:
- Acidic (pH < 7): Substances with more hydrogen ions, like lemon juice or vinegar.
- Neutral (pH = 7): Pure water, neither acidic nor basic.
- Basic (pH > 7): Substances with fewer hydrogen ions, like baking soda or bleach.
Importance of pH in Distilled Water
- Water Quality: The pH of water affects its taste, safety, and how well it supports aquatic life.
- Health & Medicine: Blood pH must remain stable (around 7.4) for proper body function.
- Agriculture: Soil pH affects plant growth and nutrient availability.
- Industrial Processes: Many industries require specific pH levels for production.
What Does a High pH Mean?
- High pH (Above 7): Indicates alkalinity. Water with a high pH may taste bitter and form scale deposits in pipes and appliances.
- Example: Alkaline water, often marketed for health benefits, typically has a pH between 8 and 9.
What Are the Risks of Consuming Unbalanced pH Water?
Drinking water with an unbalanced pH can pose several health and practical risks:
- Health Risks:
- Acidic Water (pH < 6.5):
- Tooth Decay: Acidic water can erode tooth enamel.
- Digestive Issues: It may cause irritation in the stomach and intestines.
- Metal Contamination: Acidic water can corrode pipes, leaching metals like lead and copper into drinking water.
- Alkaline Water (pH > 8.5):
- Digestive Problems: Can disrupt natural stomach acid, leading to digestion issues.
- Mineral Imbalance: Excessive alkalinity may reduce the body’s ability to absorb essential minerals.
- Kidney Stress: People with kidney conditions should avoid high-alkaline water.
- Technical and Household Risks:
- Plumbing Damage: Water with a very low or high pH can damage plumbing by corroding pipes or causing mineral buildup.
- Appliance Wear: Dishwashers, washing machines, and coffee makers may suffer from scale buildup or corrosion.
- Aquatic Life Harm: In aquariums, improper pH can harm fish and other aquatic organisms.
Real-World Example:
- Case Study: In Flint, Michigan, acidic water corroded old pipes, causing lead contamination and a major public health crisis.
Why is pH Important?
The pH scale measures how acidic or basic a substance is. Here’s why understanding the pH of distilled water matters:
- Health Applications: Distilled water is used in laboratories and hospitals because it is free of impurities and safe for medical use.
- Scientific Research: Scientists rely on distilled water as a neutral baseline in experiments, ensuring accurate and unbiased results.
- Home Uses: People use distilled water for household tasks such as watering plants and filling aquariums to avoid mineral buildup and maintain a stable environment.
How is Distilled Water Made?
Distillation involves boiling water, capturing the steam, and condensing it back into a liquid. This process removes impurities, including minerals and salts. Read More : Water Distiller Worth It?
Comparison of Water Types by Purity
| Water Type | Process | pH Range |
| Distilled Water | Boiled & Condensed | 6.5 – 7.0 |
| Tap Water | Municipal Supply | 6.0 – 8.5 |
| Spring Water | Natural Source | 7.0 – 8.5 |
| Mineral Water | Mineral Enriched | 7.0 – 8.5 |
Why is Distilled Water Not Always pH 7?
While distilled water is initially neutral, several factors can alter its pH:
- Exposure to Air: Distilled water absorbs carbon dioxide from the air, forming carbonic acid, which lowers its pH.
- Storage Container: Plastic containers may leach chemicals into the water, altering its pH.
- Storage Duration: The longer distilled water is stored, the more its pH can change due to environmental exposure.
pH of Distilled Water vs. Tap Water
Distilled water is neutral, while tap water’s pH can vary based on its source and treatment.
Example:
- Distilled Water Sample (Lab Test): pH 7.0
- Tap Water from New York: pH 7.2 (treated with chlorine to disinfect)
- Tap Water from California: pH 8.1 (contains natural minerals that increase alkalinity)
Applications of Distilled Water by pH
- Medical Use: Distilled water is clean and safe for medical procedures, such as sterilization and wound cleaning.
- Scientific Experiments: Its neutral pH ensures accurate measurements and reactions in laboratory experiments.
- Car Batteries & Appliances: It prevents mineral buildup in sensitive devices like car batteries and humidifiers.
- Skin Care & Cosmetics: Due to its purity, distilled water is gentle on the skin and is often used in cosmetic products.
How to Measure the pH of Distilled Water
You can measure pH using:
- pH Strips: Simple and affordable, these strips change color based on the water’s pH level.
- Digital pH Meter: A more precise tool that displays the exact pH value on a digital screen.
- Chemical Test Kits: Common in laboratories, these kits offer highly accurate readings through chemical reactions.
By understanding the pH of distilled water, you can make better decisions about its uses in everyday life, science, and health-related tasks.